CH3cooh Kore Tae Ahumahi me te Kai 75% 99.8% Waikawa Aitiki
Me ā mātou hangarau matua, tae atu ki te wairua auaha, te mahi tahi, ngā painga me te whanaketanga, ka hanga e mātou he heke mai angitu me tō pakihi whakahīhī mō te CH3cooh Kore Tae Ahumahi me te Kai 75% 99.8% Acetic Acid, Ko te kaupapa matua o tā mātou whakahaere he whakarato hua kounga teitei, ratonga ngaio, me te whakawhitiwhiti kōrero pono. Nau mai haere mai ki ngā hoa tata katoa ki te tuku i te hoko whakamātautau hei hanga i tētahi whanaungatanga pakihi mo te wā roa.
Me ā mātou hangarau matua, me te wairua auaha, te mahi tahi, ngā painga me te whanaketanga, ka hanga tahi mātou i tētahi heke mai whai rawa me tō pakihi whakahīhī. Ka hokona whānuitia ā mātou taonga ki Ūropi, USA, Rūhia, UK, Wīwī, Ahitereiria, Middle East, Amerika ki te Tonga, Awherika, me te Tonga-mā-rāwhiti o Āhia, me ētahi atu. E tino mōhiotia ana ā mātou taonga e ō mātou kiritaki mai i te ao katoa. Ā, e whakapau kaha ana tā mātou kamupene ki te whakapai tonu i te whai huatanga o tā mātou pūnaha whakahaere kia nui ake ai te pai o te kiritaki. E tino tumanako ana mātou kia ahu whakamua tahi mātou me ō mātou kiritaki, kia waihangahia he heke mai whai hua mō te katoa. Nau mai ki te whakauru mai ki a mātou mō te pakihi!










 Tātaihia te nui o te waikawa acetic hei ōrau papatipu (X) mā te whakamahi i te tātai:
Tātaihia te nui o te waikawa acetic hei ōrau papatipu (X) mā te whakamahi i te tātai:
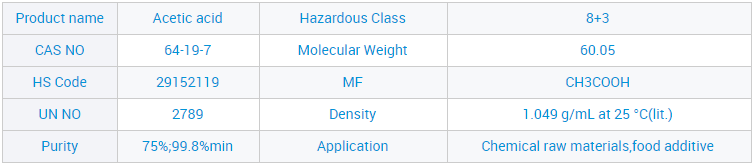
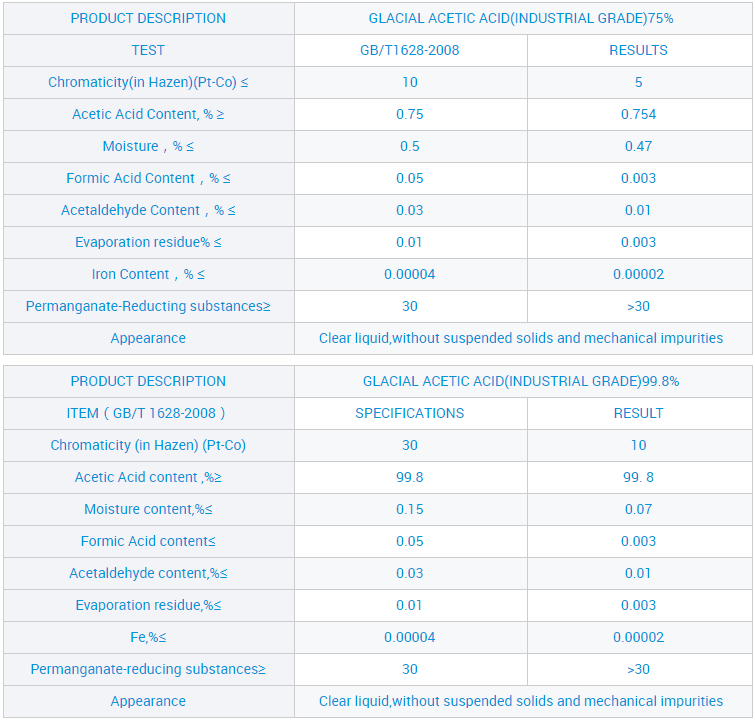
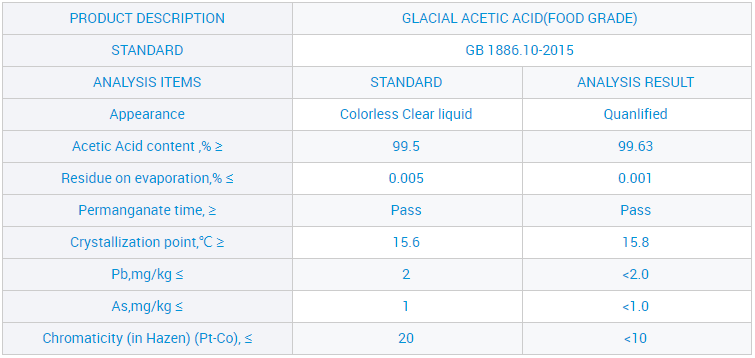
Waikawa Āketi me te Waikawa Āketi 99.8% X = (c × V × 60.05 × 10⁻³) / m
Kei hea:
V = te rōrahi o te konutai hauwai i pau (mL)
c = te kukū tuturu o te konutai hauwai (mol/L)
m = te papatipu o te tauira (g)
60.05 = te papatipu molar o te waikawa acetic (g/mol)
Waikawa Ākiti me te Waikawa Ākiti 99.8%











